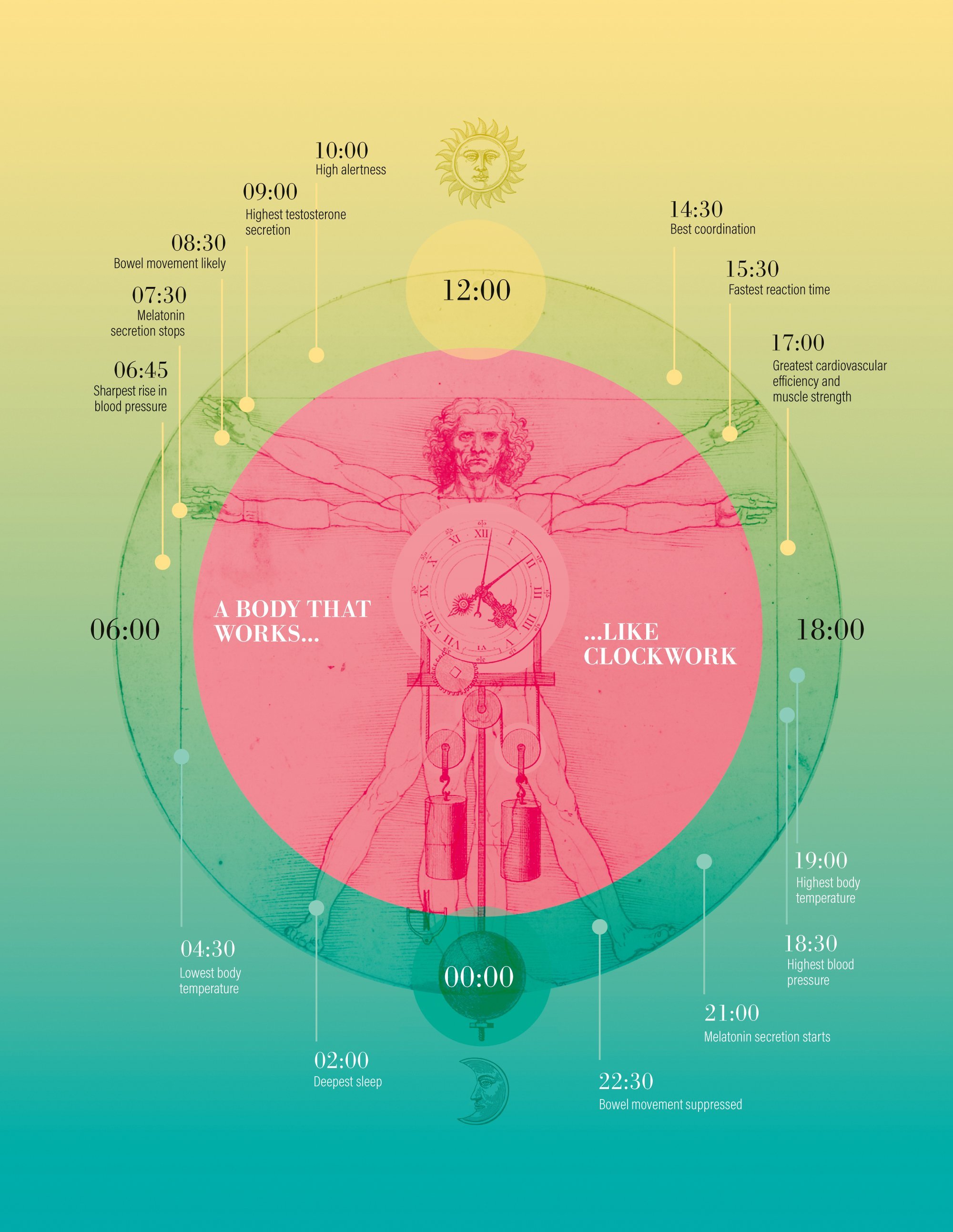
here are night owls and early birds. There are heavy sleepers and power nappers. But we all have something in common: an internal clock that keeps us following a hidden rhythm. When we wake up, our bodies stop producing melatonin (the sleep hormone), raise our blood pressure and drive us to the toilet. This is followed by a spike in testosterone and a high level of wakefulness. The afternoon gives us good coordination, fast reaction times and a high capacity for physical effort. Our temperature peaks in the early evening, before melatonin production kicks in and sends us off to bed.
An internal ticking clock
The conductor of this daily – or circadian – cycle is our internal clock, a combination of neurological and cellular mechanisms that measure the passage of time. It first serves to organise the many physiological processes that need to take place sequentially, in the liver for example. Second, it helps our bodies anticipate events in our environment that are influenced by the daily rhythm, like the mouse that needs to get in its burrow before the hawk starts hunting. “Evolution has thus selected increasingly precise biological clocks,” notes Christian Cajochen, Director of the Chronobiology Centre at the Psychiatric Clinic in Basel.
The body has two closely interwoven clock systems to synchronise its activities. On the one hand, each of our trillions of cells has its own metronome, a discovery made in the late 1990s by Ueli Schibler at the University of Geneva. These clocks work by means of a cellular feedback loop. A specific gene known as the period gene produces the PER protein. As the protein accumulates, it blocks the gene and thus its own production. As the concentration of PER falls, the gene starts to produce it again, and the cycle begins again after a period of around twenty-four hours. The exact workings are of course more complex, involving several other proteins and genes, and the discovery was awarded the Nobel Prize for Medicine in 2017.
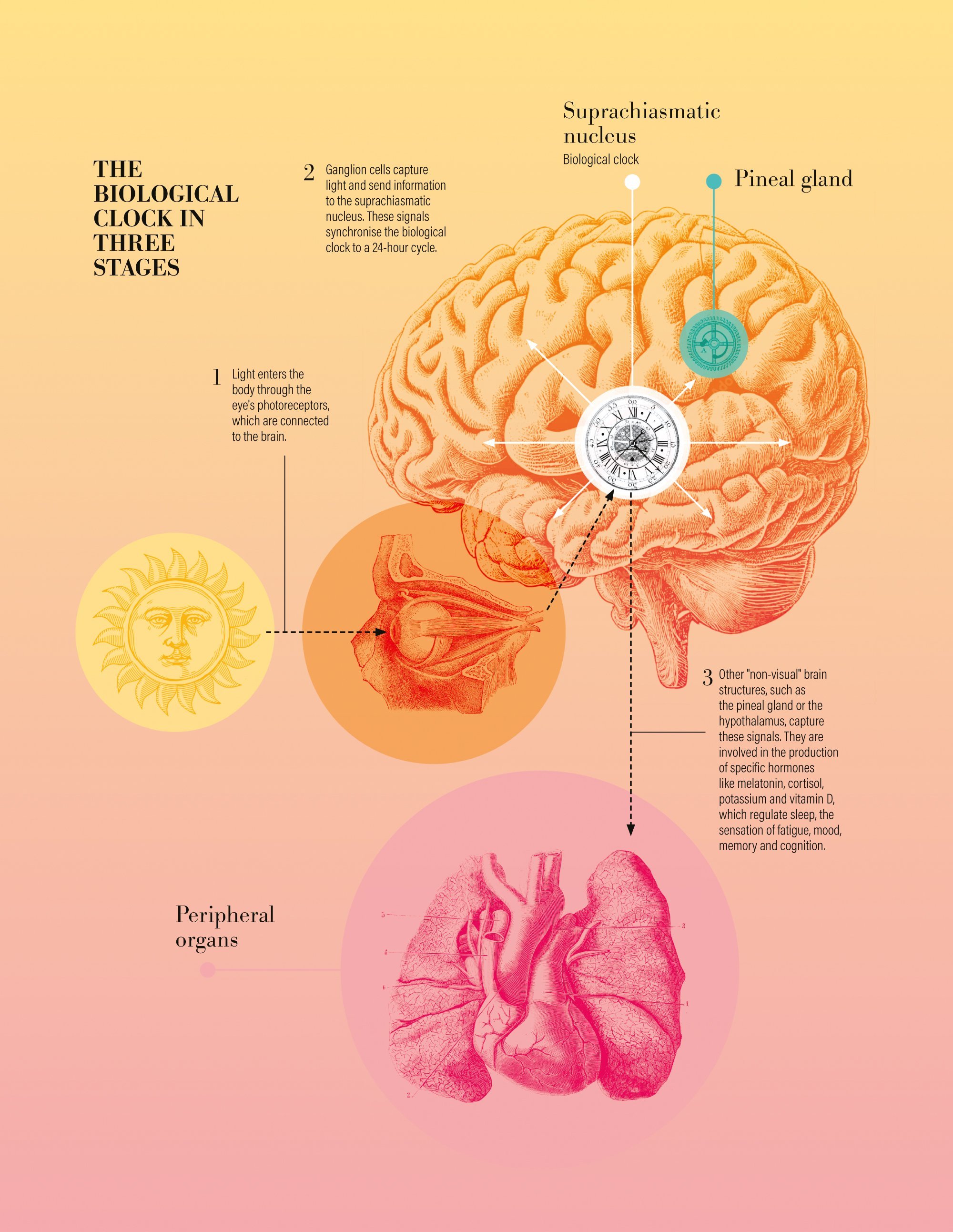
A master clock in the brain
In parallel, a small area of the brain acts as a master clock that regularly synchronises the times given by our multitude of cellular clocks. This area – the suprachiasmatic nucleus – is synchronised with the length of a day on Earth thanks to information about ambient light received directly from the eye, continues Christian Cajochen. This information is collected by photosensitive ganglion cells in the retina, which complement the two other types of photoreceptors in the eye (rods for monochromatic vision in low light and cones to distinguish colours). These ganglion cells have the unique task of informing the brain of the time of day, a role that was only identified around forty years ago. The nerve signals they transmit to the brain tell it, among other things, how long it will remain bright after a period of darkness, which is directly related to solar time.
Without this synchronisation by daylight, our internal clock tends to be delayed. Experiments carried out in caves and isolated rooms have shown that participants lengthen their daily rhythm by around fifteen to thirty minutes, enough to cause a complete shift after a few weeks. But this is an average, with some people seeing their biological day shorten when isolated from daylight.
When a day is longer than twenty-four hours
In some people, Christian Cajochen notes, their internal clock is out of sync with the terrestrial day, even in the presence of daylight. “Our team treated a patient who had a rhythm of 25.5 hours. As these delays accumulate, he regularly finds himself out of step with the rest of society and has for example to sleep in the middle of the day. His rhythm does not adapt to daylight and continues as if he were in a different time zone.Our hypothesis is that the problem lies in his photosensitive ganglion cells.”
This type of syndrome also occurs in people who are completely blind, for example following cancer leading to removal of the eyes, but not in most visually impaired people whose ganglion cells are functioning well. Such a mismatch also exists in people who work nights or change time zones frequently, such as airline crews. “Living out-of-sync has major consequences for health, in particular with sleep disorders and depression,” explains the biologist. “Timed exposure to light as well as taking melatonin can help synchronise the biological clock with social time. This can sometimes alleviate or even stop a depression caused by a disturbed circadian rhythm.”
Chronomedicine, or time that heals
A disrupted circadian rhythm could also play an important role in problems of overweight and diabetes, adds Charna Dibner, a professor at the Faculty of Medicine at the University of Geneva and a specialist in cellular clocks. Disruption of the internal clock could have a negative impact on the regulation of insulin production, a hormone that plays a major role in metabolism and diabetes.
This type of research has opened up the new field of chronomedicine, which focuses on the temporal aspects of diseases and therapies. “When we are consulted about being overweight, we shouldn’t just ask: what do you eat? But also: when do you eat?” says Dibner. Our internal clock thus represents an important therapeutic target, but one that is still underused, according to her. Chronobiology centres are beginning to emerge in Europe, but most doctors do not yet consider their patients’ circadian rhythms.
The pharmaceutical sciences are also beginning to realise the potential offered by the human body’s circadian cycle. Chronopharmacology seeks to determine the right time for administering therapeutic substances in order to maximise their effectiveness while minimising their toxicity. “Our team aims to study the impact of timing on chemotherapy, which attacks both cancerous and healthy tissue,” explains Charna Dibner. “We hope to be able to reduce the side effects by administering the substance when the detoxification processes taking place in the liver are at their most intense.”
Light that doesn’t wake you up
Regular exposure to daylight represents a therapeutic approach that is as simple as it is inexpensive. Where daylight is not available, artificial light can help. Oliver Stefani from the Lucerne University of Applied Sciences has worked with the Basel team to develop computer screens that can be used to stimulate ganglion cells, or not. This can be used to trick the brain into believing that it is day or night, at will. “The night mode on a mobile phone already acts on such mechanisms, but it creates an orange colour that can be unpleasant,” explains Oliver Stefani. “In contrast, our screen can produce two types of white light that appear identical to the human eye, a phenomenon known as metamerism.”
In the first mode, the turquoise part of the light spectrum is produced from blue and green, which stimulates the ganglion cells only moderately. In this case, the circadian rhythm of someone working at a computer at night will not be disturbed. In the second mode, turquoise LEDs generate the colour directly, exciting the ganglion cells and increasing the user’s state of wakefulness. This can disrupt the user’s biological clock, but such enhanced wakefulness can be valuable in professions such as air traffic control or power plant surveillance. “People who work at night often drink caffeinated energy drinks to stay awake,” the engineer continues. “Our prototype would enable them to choose the mode that suits them best, without the need for any substances and in a more controllable way.”
There are many ways for us to better synchronise our circadian rhythms with social time: when we go to bed, natural or artificial light, taking medicines such as melatonin, or changing the time we eat. But it’s also about respecting our own rhythm, argues biologist Charna Dibner: “I often asked my kids to respect the family mealtimes. But then I became a chronobiologist and realised that it’s better if our eating times match our chronotype. So I have one recommendation for good health: synchronise to your internal clock!”




















